
Frequently Asked Questions
General
How many samples can I run with a kit?
The number of samples you can run with a kit will be dependent upon how many samples you analyze with each run. To ensure accuracy and reliability of data, Gold Standard Diagnostics strongly encourages our customers to use our microtiter ELISA plates according to two recommendations:
1. A full set of calibration standards and quality controls should be run every time samples are analyzed (regardless of frequency).
2. Calibration standards, quality controls and samples should be run in duplicate.
For example, our Microcystins/Nodularins ADDA kit includes six calibration standards (including Standard 0), one quality control and a Laboratory Reagent Blank, for a total of eight known. Running each of these in duplicate wells consumes 16 total wells, which leaves 80 wells left for sample analysis. This equates to 40 samples run in duplicate. If 40 samples are not analyzed on the initial run, the remaining wells and reagents can be stored per the instructions on the kit user’s guide and be used later. However, keep in mind that 16 more wells will be consumed for the calibration standards and controls the next time the kit is ran, which means fewer wells available for sample analysis.
What are the acceptability requirements for your assays?
There are four main criteria that should be assessed when evaluating your data:
1. Your standard curve R2 must be ≥ 0.98.
- If this condition fails, all results must be discarded and the assay must be rerun.
2. % CVs of Absorbance Values:
• Standard Absorbance Values must be ≤ 10%.
• One absorbance CV may be between 10-15% as long as the rest are < 10%.
• Control and Sample Absorbance CV values should be ≤ 15%.
• If the standard absorbance criteria are not met, the assay should be rerun as this could impact the curve fit and produce inaccurate results.
• If the sample criteria are not met for a single sample, you can accept your results for the passing samples and rerun the failing sample with your next run.
3. Control concentrations should be within their acceptable ranges.
- For assay and control specific ranges, please reference the user’s guide found in the kit of your assay. If this condition fails, it may indicate a problem with the standard curve. Results should be discarded and rerun.
4. Standard 0 Absorbance Value should be between 0.800 and 3.000.
- If the absorbance value of standard 0 is outside of the range, there may be an issue with one of the assay’s reagents or one of the steps in the procedure was not executed properly. If this condition fails, all results must be discarded and the assay must be rerun.
Should I get the microtiter plate version or magnetic particle version of the assay I am interested in?
While both approaches will provide excellent results, most of our customers experience greater success with our microtiter plate format. There are several reasons for this:
1. Most tube readers only read one tube at a time. This requires the analyst to spend several minutes reading each sample individually.
2. The tube reader we offer does not connect to a computer and does not come with software that can generate the results for you. The reader generates absorbance values that must be plugged into a solver to generate results. This increases total analysis time and increases the likelihood of human error.
3. Most microtiter plate readers are able to read an entire plate at once. The 4303 plate reader not only reads the entire plate in a matter of seconds, but also comes with software that calculates results and generates a report.
Most assays are available in the microtiter plate format, but not all assays are available in the magnetic particle format. Most customers prefer to purchase the equipment for the microtiter plate formats because this gives them added flexibility should new testing needs arise in the future.
Do I have to run my standards/controls/samples in duplicate?
Running all samples, as well as quality controls and calibration standards, in duplicate is strongly encouraged. You may experience a certain degree of variability in the absorbance due to certain environmental factors that are outside of your control (humidity, temperature, air flow, etc.). Human error, such as improper pipetting or washing, can also cause issues with individual wells. If samples are run in only a single well, it is impossible to know if a well was adversely affected at any point during the analysis. When samples are run in duplicate, however, it allows the user to determine consistency between replicates. This is done by calculating the Coefficient of Variation (%CV) between the sample wells. When the %CV is ≤ 15%, it shows good correlation between wells, which indicates the result is accurate.
Can you guarantee a specific expiration date for a kit I want to order?
Specific expiration dates cannot be guaranteed for our kits, as the expiration dates are assigned during manufacturing. Customers are welcomed to inquire about available expiration dates for the kits that are currently in inventory prior to ordering. If one expiration date is preferred over another, please let the sales representative taking the order know so they can specify the desired lot with the order.
I only have a few samples; will Gold Standard Diagnostics run them for me?
Gold Standard Diagnostics is a manufacturing facility. While we do have a research and development team onsite, we do not offer commercial testing. However, there may be a commercial testing lab in the Eurofins lab network that can meet your needs. For contact information for a specific laboratory, please refer to the Eurofins group directory.
I am having trouble interpreting my test strip results, can you help?
When evaluating our strip tests, it is important to make sure you are reading them at the appropriate time. For most of our tests, results should be read after removing the strip from the conical vial and allowing it to lay flat and dry for 5-10 minutes. (The appropriate dry time will be specified in the individual procedures). After the drying period, a control line should be easily visible. The control line is the line that will form closest to the blue and white adhesive part of the strip, opposite from the end that is placed into the sample. Next, compare the intensity of the test line (this is the line nearer to the part of the strip that is placed into the sample) to the intensity of the control line. DO NOT COMPARE THE STRIP TO THE DIAGRAM IN THE PROCEDURE.
• If the test line appears darker or in equal intensity to the control line, this indicates a sample that has a lower concentration than the strip detection limit.
• If the test line appears lighter than the control line, this indicates that the sample is positive and has a higher concentration than the strip detection limit. A test line that gets lighter in intensity, indicates a higher concentration of toxin in the sample.
• If there is no test line present, this indicates that the sample has a concentration at or above the highest concentration the test can detect.
For more information and examples of various concentration levels, please refer to the
Test Strip Interpretation Guide
Important: If your sample requires a dilution or extraction, this will change the range of detection for the strip. Please refer to the specific sample prep bulletins for adjusted range.
Algal Toxins
Can you provide guidance as to how I should be collecting and storing my algal toxin samples?
Please refer to our Quick Reference Guide for Cyanotoxin Sample Collection
What are the pH and methanol tolerance levels for the Gold Standard Diagnostics algal toxin kits?
|
Assay |
% Methanol |
pH |
| Microcystins ADDA | 5 | 4-11 |
| Microcystins SAES | 1 | 3-11 |
| Microcystins DM | 20 | 4-11 |
| Saxitoxin | 20 | 3-11 |
| Cylindrospermopsin | 20 | 4-11 |
| Anatoxin-a | 2.5 | 5-7 |
* Please note: samples being tested for Anatoxin-a should have their pH adjusted immediately upon collection to prevent toxin degradation.
** Sample pH may be adjusted using 1N HCl and 1N NaOH
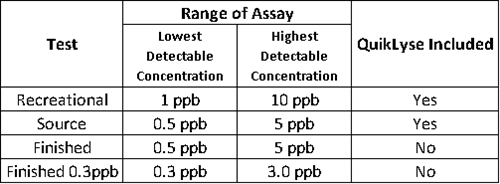
What are the differences between the Microcystins test strips? Which kit should I buy?
As a basic rule of thumb, if you are testing water that has been treated with chlorine, you should use one of the finished drinking water test kits. Finished water samples will not need to undergo a cell lysing process so these kits will not contain QuikLyse. Since source and recreational waters have not been treated, cells will need to be lysed prior to testing to obtain a total toxin concentration (internal and external cell toxins). These kits include QuikLyse to achieve the lysing step. The kits also have different reporting limits that should be considered when choosing a kit.
Important
* To screen chlorinated water samples for total Microcystins, samples must be preserved (quenched) with sodium thiosulfate at the time of collection and should be manually lysed (freeze/thaw method) prior to testing. If the chlorine is not quenched prior to analysis, it will interfere with the assay.
* If using the Recreational or Source kits for finished drinking water, QuikLyse reagents must still be used. Not using the reagents in the procedure will adversely affect the performance of the test, producing inaccurate results.
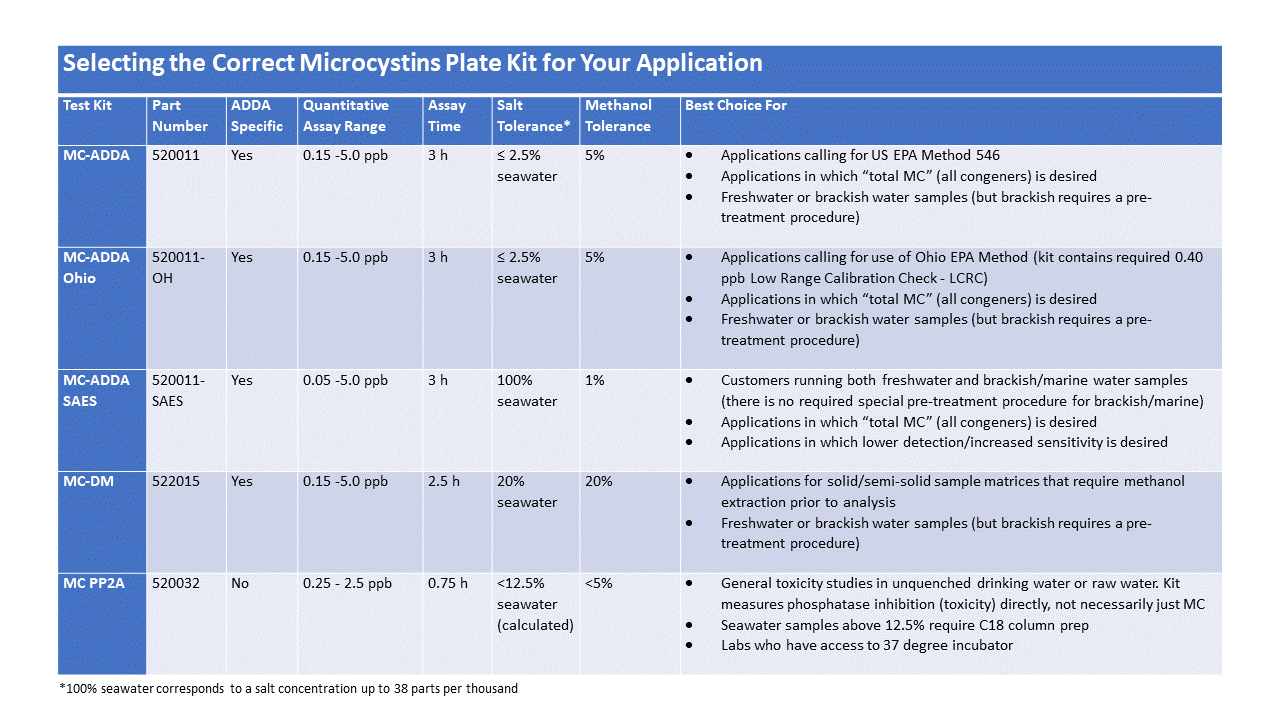
What are the differences between the various Microcystins ELISA kits?
Instruments & Software
I am using the Excel solver; can you help me generate my results?
Please refer to the Excel Solver User Guide for step by step instructions.
How do I schedule a PM for my CAAS?
Please contact support.abraxis@us.goldstandarddiagnostics.com for assistance. Provide the serial number along with the desired timeframe for the service.
Support has asked me to send them a pack file. How do I do that?
Instructions for packing and sending a file can be found Here